Bacteria chemically alter coolants, destroying lubricants and corrosion inhibitors, while releasing corrosive acids and salts into the fluid.
There are more than 2,000 species of bacteria on earth, and there is virtually no place that one species or another does not call "home." These omnipresent microorganisms represent some of the most harmful and most beneficial forces in nature.
The "good" bacteria that line human intestines help turn food into useable fuel, while the bacteria that cover our skin help keep it healthy and strong. However, bacteria also have a "nasty" side. Different strains are responsible for a number of illnesses, from the common flu, to strep throat, to highly publicized contaminations of foodstuffs by e-coli bacteria.
Users of water-miscible cutting and grinding fluids know that bacteria can literally "reek" havoc on coolants, killing fluid performance and making a big stink in the process. Bacteria chemically alter coolants, destroying the lubricants and corrosion inhibitors that make coolants work, while releasing corrosive acids and salts into the fluid. Bacteria prefer water-miscible fluids, because they need water to grow. Without moisture, most cannot reproduce and multiply once introduced to a new environment. Furthermore, most bacteria (aerobic bacteria) need oxygen to survive. In the typical coolant system, there is no shortage of oxygen. As coolants cycle through, they come into contact with the life-giving gas at the surface of sumps and holding tanks. Fluids pick up oxygen as they are ejected from the nozzle and just about anywhere else coolant is exposed to the air.
How Fluids Get Contaminated
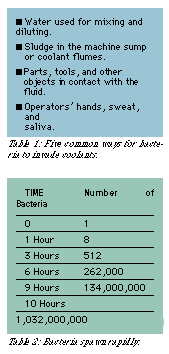
Bacteria find their way into fluids through a variety of ways (Table 1). For instance, there are bacteria in the water used for diluting and mixing. They thrive on wet parts and lurk in the air. Bacteria from an operator's hands, sweat, and saliva also get into coolants. Surprisingly, there are people who find it easy to confuse coolant sumps and holding tanks with toilets or food disposal units. Poor housekeeping practices add huge numbers of microorganisms to the system. Bacteria also live in the sludge that settles in machine sumps and coolant flumes. In short, bacteria are everywhere.
Aerobic bacteria reproduce by dividing in two approximately every 20 to 30 minutes (Table 2). If you do the math, a single bacterium that replicates itself every 20 minutes does not take long to create an entire army of clones, all working to destroy your coolant. A single bacterium that remains well-fed can produce a spawn billions strong in less than half a day.
Aerobic Bacteria
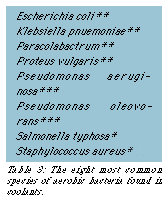
There are fewer than a dozen species of bacteria commonly found in water-miscible fluids (Table 3). Known as "facultative" bacteria, they prefer air (oxygen) for growing and reproducing. In the absence of oxygen, they will continue to survive, but grow very slowly until oxygen is reintroduced. Once they can "breathe" again, facultative bacteria will resume reproduction at their usual break-neck pace.
Typically, the two most troublesome aerobic bacteria are pseudomonas oleovorans and pseudomonas aeruginosa. Pseudomonas oleovorans prefer oil as a food source, so they tend to grow rapidly in those machines that leak substantial amounts of lubricating and hydraulic oils. Consequently, everything should be done to reduce tramp oil contamination. Contaminant oils and greases that do make it into coolant should be skimmed off the surface or centrifuged out of the fluid.
Pseudomonas aeruginosa can live on practically anything: minerals in the water, coolant concentrate, discarded food, oils, and so on. These two species of bacteria are commonly found in virtually all water-miscible fluids in use; and because they can call on these highly successful defense mechanisms, they also are two of the most difficult bacteria to kill.
Anaerobic Bacteria
Another class of bacteria, known as anaerobic, grow best in the absence of oxygen. This type of bacteria grow much more slowly than the aerobic, dividing in two approximately every four hours. Additionally, some anaerobic bacteria are sulfate-reducers, which liberate hydrogen sulfide gas as a growth byproduct. The gas is offensive to humans and detectable by the human nose in the parts-per-billion range.
Anaerobic bacteria will usually not grow until the fluid has first been attacked by aerobic bacteria and the oxygen is depleted. Some components of coolant-emulsifier systems are naturally toxic to anaerobic bacteria; that's why adding fresh coolant makeup to stinky coolant will "freshen up" the mix for only a short time.
Fluid Performance
Lubricating and anti-corrosion properties are built into cutting-fluid concentrates. When they are mixed with water at the proper concentration and well maintained, they will lubricate and cool in the cutting zone while leaving a film of corrosion inhibitors on the finished piece. However, when subject to bacterial degradation, the lubricants function at a reduced level so that tool and wheel life are reduced and workpiece corrosion can take place.
As mentioned above, growing bacteria produce acids and salts that cause corrosion. The more rapidly bacteria grow, the faster they alter the fluid. That makes the rate of bacterial growth an extremely important factor to limit. If the rate of bacterial reproduction can be limited, the harmful effects of their growth can be substantially reduced.
Controlling Bacterial Growth

As pervasive and stubborn as bacteria may be, there are ways to address the problem (Table 4). One of the most important methods of keeping bacterial growth in check is to be sure that the cutting-fluid manufacturer you select uses the finest, highest purity raw materials for its products. Bacteria have very specific appetites; some materials are much better food for them than others.
Fluid manufacturers that understand the biology of microorganisms select raw materials that bacteria do not find particularly appetizing. Coolants formulated with materials bacteria find undesirable are, by nature, less vulnerable to bacterial attack.
Germicides can be helpful in preventing or retarding bacterial degradation. However, very few germicides are truly effective in coolant systems, and they must be used with great care. Moreover, those biocides that have proven to be most effective often cannot be used in all types of coolants.
When coolants require the addition of biocides, it's important to carefully follow the supplier's recommendations. Do not add too little or too much coolant - too little can actually stimulate microbial growth, while too much can cause operator skin irritations. In short, never add any chemicals to your coolants without specific instructions from your coolant supplier.
It is extremely important to practice good housekeeping. In fact, this is the best way to effectively control bacterial growth. It does little good to put fresh coolant into a dirty machine if you're only going to suck out the old before putting in the new. This practice provides fresh food for the bacteria that are in the swarf and sludge that remain in the machine sump, on machine surfaces, and in the coolant-circulating systems. If a machine is thoroughly cleaned with a proper cleaner, thoroughly rinsed, and then filled with clean, fresh fluid, the fluid will last four to six times longer than it would in a machine that is simply "sucked out" and recharged with fresh coolant.
Central Systems
Central systems generally involve quantities of fluid three to five times the amount used for an individual machine. As a result, if the bacteria in a central system grow at the usual rate, the proportionately smaller addition of fresh makeup will give the bacteria a greater period of time in which to break down the coolant than would be the case with individual machines.
Further compounding the problem, bacteria tend to settle to the bottom of tanks. In any system where the fine metal pArticles and other silt settle, so do the bacteria. This combination allows the anaerobic bacteria to achieve full growth potential, since they have plenty of food and are far away from the oxygen at the fluid surface. Consequently, it is usually more difficult to control bacterial growth in large central systems than it is in individual machine sumps. However, proper cleaning of such systems with each coolant change and good filtration to prevent sludge accumulation can help keep bacterial growth under control.
Water-miscible users should use a high-speed disc bowl centrifuge to continuously remove tramp oils and very fine particulate matter in central systems. Continual removal of these contaminants will greatly reduce bacterial growth rates.
Beware of Fungal Attack
Besides bacterial growth, there is another whole group of microorganisms that can infect coolants. Collectively, they are referred to as fungus which, in reality, is a combination of molds and yeasts.
While bacteria are essentially animal-like and grow rapidly as discrete cells or pArticles within the fluid, fungus are plant like, growing in layers on surfaces (e.g., inside pipes). Bacteria and fungus compete for the same food sources, and because of bacteria's animal-like nature, they can generally out-compete fungus. As a consequence, if a coolant system has a moderate (105-106), stable (neither rapidly increasing nor decreasing) bacterial population, the bacteria will generally control fungal growth. Conversely, if a coolant system has a consistently low (<104) bacterial population and the fluid will allow fungal growth (soluble oils tend to support bacterial growth and chemical [synthetic] fluids tend to support fungal growth), the fluid will likely develop a significant fungal population eventually.
Fungus can be even more of a problem than bacteria because fungal accumulations can interfere with machine functioning and can plug coolant filters and piping. While there are a number of effective microbiocides that can be added to coolants to control bacteria, there are very few effective fungicides for use in coolants.
In short, there is no simple solution to microbial problems in coolants. The best a manufacturer can do is formulate coolants to be as bioresistant as possible. End users must apply them at the recommended concentration in machines that are periodically cleaned and rinsed, while taking steps to minimize tramp-oil contamination. In addition, operators must be trained to prevent contamination of the coolants by materials that support bacterial and fungal growth.
When properly maintained, fluids last much longer today than they did just 10 years ago. However, the search for an ever-longer life cycle continues, because the market demand is for fluids that will never be bacterially degraded and, therefore, never require disposal. While this is the goal, it would be wise to remember the words of Louis Pasteur: "In the end, the microbes will win."
About the Author
William Sluhan is chairman of the board of directors and CEO of Master Chemical Corp., Perrysburg, OH. He has more than 30 years of experience in the design, implementation, and evaluation of coolant-management
Related Glossary Terms
- centrifuge
centrifuge
Filtering device that uses a spinning bowl and the differences in specific gravities of materials to separate one from another. A centrifuge can be used to separate loosely emulsified and free oils from water-diluted metalworking fluid mixes and to remove metalworking fluids from chips.
- concentrates
concentrates
Agents and additives that, when added to water, create a cutting fluid. See cutting fluid.
- coolant
coolant
Fluid that reduces temperature buildup at the tool/workpiece interface during machining. Normally takes the form of a liquid such as soluble or chemical mixtures (semisynthetic, synthetic) but can be pressurized air or other gas. Because of water’s ability to absorb great quantities of heat, it is widely used as a coolant and vehicle for various cutting compounds, with the water-to-compound ratio varying with the machining task. See cutting fluid; semisynthetic cutting fluid; soluble-oil cutting fluid; synthetic cutting fluid.
- grinding
grinding
Machining operation in which material is removed from the workpiece by a powered abrasive wheel, stone, belt, paste, sheet, compound, slurry, etc. Takes various forms: surface grinding (creates flat and/or squared surfaces); cylindrical grinding (for external cylindrical and tapered shapes, fillets, undercuts, etc.); centerless grinding; chamfering; thread and form grinding; tool and cutter grinding; offhand grinding; lapping and polishing (grinding with extremely fine grits to create ultrasmooth surfaces); honing; and disc grinding.
- swarf
swarf
Metal fines and grinding wheel particles generated during grinding.
- tramp oil
tramp oil
Oil that is present in a metalworking fluid mix that is not from the product concentrate. The usual sources are machine tool lubrication system leaks.
