The commercial availability of amorphous-diamond films is good news for the metalworking industry. Limitations of the conventional cathodic-arc process previously made it difficult and expensive to deposit these films on carbide and HSS cutting tools. However, improved deposition technology has made amorphous-diamond films a practical choice for nonferrous metalcutting.
Amorphous-diamond films have proven to be cost-effective coatings for tools for the milling and drilling of nonferrous materials such as graphite, wrought aluminum, die-cast aluminum, and carbon composites. Priced 10 to 20 times lower than chemical-vapor-deposited (CVD) diamond films, amorphous-diamond films have a low coefficient of friction, high hardness, good abrasion resistance, and good thermal and chemical stability (Table 1). The film’s low friction coefficient helps the tool run cooler and prevents workpiece material from adhering to the film. Its hardness and abrasion resistance help keep cutting edges sharp. All these properties help extend tool life and improve machining productivity in nonferrous-metalcutting applications.
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Table 1: Comparison of the properties of various diamond films with the properties of natural diamond. |
Amorphous Advantages
Amorphous-diamond films are part of the nonhydrogenated class of amorphous carbon-based films. Unlike diamond-like carbon (DLC) films, which are synthesized using hydrocarbon gas, amorphous-diamond films can be synthesized without the use of hydrogen or any other reactive gas.
Both types of carbon-base films are amorphous, because no long-range order in the structure of the carbon atoms can be observed. While DLC films are mostly composed of weak carbon-hydrogen bonds, amorphous-diamond films are made up of strong carbon-carbon bonds. The bonding of carbon atoms in the graphite used to produce amorphous-diamond films is referred to as sp2. By maintaining a high level of ion energy, the evaporated carbon can be condensed to sp3 bonds as the film is being grown. By definition, sp3-bonded carbon films are tetrahedrally bonded. Each carbon atom is bonded to four other carbon atoms; thus, the sp3 bond is robust in all directions.
This sp3 bonding is typical of natural diamond. Amorphous-diamond films have very high sp3 fractions—in the range of 80% to 90%—according to measurements via energy-electron-loss spectroscopy. Studies have shown that sp3 fractions are directly related to film hardness. The hardness of amorphous-diamond film has been measured in the range of 70 to 90 GPa, which is about the same as that of CVD diamond film. Abrasion resistance is also comparable to that of CVD diamond film.
Very low friction coefficients in the range of 0.10 to 0.15 have been measured for amorphous-diamond films by pin-on-disc/ball-on-disc tests against steel in ambient conditions. Such a low coefficient of friction can be achieved without the extensive polishing or lapping that CVD diamond film requires.
Amorphous-diamond films adhere well to almost any carbide grade. They can be applied to the most commonly used substrates at relatively low temperatures (20° to 150° C) via physical vapor deposition (PVD). By contrast, the quality of a CVD diamond film’s adhesion depends on very specific choices of carbide substrates. Standard- and micrograin-carbide grades containing 6% cobalt or more cannot be successfully coated with CVD diamond. CVD diamond films are deposited at high temperatures (900° C), and the nucleation takes place on the tungsten-carbide grains. Under CVD diamond growth conditions, the cobalt in tungsten-carbide tools acts as a catalyst for the formation of weakly bonded carbon. This graphitic layer essentially prevents the adhesion of the diamond to the substrate.
Amorphous-diamond films not only offer flexibility in substrate selection; they also allow tool users to select from a variety of geometries to optimize a particular application. Because the coating zones in CVD reactors are very narrow, the process favors flat, one-sided tools with simple geometries. With CVD diamond technology, only one side of an insert and its flanks can be coated at one time. Due to the high processing temperature and other considerations, it is not feasible to reprocess the insert and coat the other side.
For example, the amorphous-diamond film was tested on a neutral-rake, double-side carbide insert in the machining of graphite. Although one side of this insert lasted only 75% as long as a single-side insert coated with CVD diamond, the amorphous-diamond-coated insert offered two more cutting edges. Therefore, the amorphous-diamond-coated insert lasted 150% longer than the CVD diamond-coated insert.
Among the PVD processes used to produce amorphous-diamond films, cathodic-arc evaporation provides films with the highest hardness and properties most like diamond. However, enhancements were necessary to make the conventional cathodic-arc process practical and economical for the deposition of amorphous-diamond films on cutting tools.
Enhanced Arc
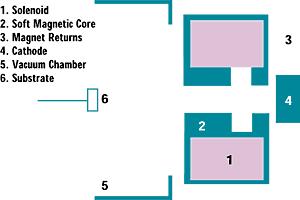 Figure 1: Schematic of the enhanced arc apparatus used to evaporate the solid-graphite cathode. |
|
The conventional cathodic-arc deposition process uses an electric arc to evaporate coating material and deposit it on the tool substrate. The deposition stage is carried out in a vacuum chamber. A direct-current arc discharge is ignited by an electrical contact between a mechanical trigger and the cathode, which is made of the coating material. When the arc strikes the cathode, the material is evaporated directly from solid to vapor. As the evaporated material passes through the arc, it becomes ionized.
The success of this process depends on the uniform erosion of the cathode material over its surface area. When metallic coatings such as titanium nitride (TiN) are deposited using conventional cathodic-arc evaporation, uniform erosion is not a problem. As the metal evaporates, the temperature of the tiny arc spot increases. The rise in temperature of this single spot causes the electrical resistivity to rise, which forces the spot to move to a portion of the cathode where the resistivity is lower; hence, the metal cathode is evenly eroded.
However, the graphite cathode used to produce nonhydrogenated carbon films does not erode evenly. When graphite is evaporated, the electrical resistivity decreases as the temperature of the arc spot increases. Thus, the arc stays in the same place, and the spot quickly bores a hole in the material. Consequently, long coating cycles cannot be maintained, making the conventional cathodic-arc process uneconomical.
Another drawback of using the conventional arc process to deposit metallic or nonmetallic films is that it produces macropArticles of the coating material during evaporation of the cathode. These pArticles can become trapped in the growing film on the substrate.
Researchers have enhanced the arc-evaporation process by creating an electromagnetic field in front of the cathode. This magnetic field steers the arc spot across the cathode’s surface, reducing the dwell time at any given point. This allows for uniform cathode erosion and sustained coating cycles. It also significantly cuts down on the production of macropArticles of coating material.
Figure 1 illustrates the enhanced arc system. A solenoid is mounted between the cathode and the vacuum chamber. Steel magnetic returns are mounted around the coil to contain the magnetic field. A soft magnetic core with an air gap is mounted inside the solenoid to optimize the shape and strength of the magnetic field. The arc is confined on the face of the cathode by the magnetic field produced by the solenoid and core.
The carbon plasma stream is magnetically filtered and channeled through the solenoid, magnetic returns, and core assembly. The focusing and defocusing of the carbon plasma increases the number of collisions between energetic electrons in the magnetic field and graphitic pArticles ejected from the arc spot. This phenomenon results in the enhancement of plasma ionization, energy, and density and the near elimination of macropArticles in the deposited film.
The additional ionization also assists in the final cleaning of the substrate prior to coating. The more the plasma is ionized, the more energy is available to bombard the surface of the tool to be coated. By increasing the negative voltage on the tool surface, cleaning action is enhanced. During this phase of the process cycle, the ionized carbon atoms are accelerated toward the tool surface. Under the proper conditions, the carbon atoms either intermix into the surface or resputter off the surface. The resputtering action removes any remaining contaminants from the surface.
This high energy of the ionized plasma, combined with the high negative voltage, aids in the intermixing of carbon into the substrate. The enhanced arc process can produce a very thin zone on the tool surface where the carbon and substrate are intermixed. Because the film can be grown out of the tool surface rather than on the tool surface, this technique promotes excellent film adhesion.
The enhanced arc process has made it possible to deposit high-density, highly adhesive amorphous-diamond films on certain tools that cannot be coated effectively with CVD diamond films. For example, extremely small tool geometries favor thin, hard films. In these cases, amorphous diamond is the only practical option. It is not easy or economical to coat tools less than 0.250" in diameter with CVD diamond. When applied to microtools for the drilling and milling of circuit boards, amorphous-diamond films have yielded good results compared to uncoated carbide. The film’s low coefficient of friction allows for greater feeds without increasing tool breakage, and its hardness helps extend the life of the tools. The use of fewer tools and the increase in hole production have significantly reduced the overall cost of microdrilling and micromilling.
Amorphous-diamond films don’t always perform as well as CVD diamond films on a cost-per-piece basis, however. In some nonferrous-metalcutting applications, CVD diamond films are more cost-effective.
Diamond at Work
While both amorphous-diamond and CVD diamond films are suited for many of the same nonferrous-metalcutting applications, there are jobs for which a 20µm-thick CVD diamond film is a better choice than a 2µm-thick amorphous-diamond film. On 3-D cutting tools, it is very difficult to maintain high hardness and good adhesion of amorphous diamond if the film thickness is greater than 2µm. Despite the good abrasion resistance of amorphous diamond, it is too thin for the machining of highly abrasive, large-particle materials, such as metal-matrix composites (MMCs) and high-silicon sand-cast aluminum. CVD diamond also is better suited for long-run applications where abrasive wear is the predominant failure mode.
In controlled tests, an MMC material with a particle size of 25µm was turned with two types of coated carbide inserts. The wear life of 2µm-thick amorphous diamond was 2 minutes, and the wear life of 25µm-thick CVD diamond was 35 minutes. Although the two films wore about the same on a per-micron basis, the thicker coating proved to be more suitable for this application, because the tool could remain in the cut longer. Indexing the tool every 2 minutes would not be practical.
Coating developers are finding ways to improve the performance and cost effectiveness of amorphous-diamond films for nonferrous metalcutting. They are making the films more robust, using multiple evaporators to maximize the effective coating zones in the reactors, and optimizing fixtures to maximize load densities.
Coating Commercialization
Amorphous-diamond film is entering the mainstream of metalcutting, but because of its limitations it will likely remain a niche product. Market size remains a limiting factor, because nonferrous metalcutting is less prevalent than ferrous metalcutting. Amorphous-diamond films outperform PVD metallic coatings, such as TiN, titanium carbonitride, and titanium aluminum nitride, in nonferrous metalcutting; however, the price of amorphous-diamond film is five to 10 times higher than that of PVD metallic coatings because of its small market size and limitations in the deposition process. Because PVD metallic coatings can be used in ferrous as well as nonferrous applications, their market is much bigger and economies of scale keep their cost down.
Coating developers have been exploring ways to increase the thickness of amorphous-diamond films without sacrificing hardness. Some believe that doubling the thickness of an amorphous-diamond film to 4µm would more than make up for the slight reduction in wear resistance. It also would reduce the film’s intrinsic stresses, which typically range from 4 to 10 GPa.
Cleaning and fixturing also require special attention. Temperatures must be maintained so that the substrate doesn’t get hotter than 150° C and compromise the properties of the film. If the temperature of the tool is allowed to rise over 150° C, the film begins to revert to graphite/sp2 carbon. To maintain temperatures during the coating cycle, a series of water-cooled fixtures control the temperature throughout the coating cycle.
Although a low temperature is desirable for retaining film properties, it is not ideal for cleaning tools, which is absolutely necessary before coating can begin. However, when greater emphasis is placed on cleaning tools more thoroughly outside the chamber, the need for high temperatures in the chamber is reduced.
About the Author
Richard Horsfall is president of TetraBond, a division of Multi-Arc Inc., Rockaway, NJ.
Related Glossary Terms
- 3-D
3-D
Way of displaying real-world objects in a natural way by showing depth, height and width. This system uses the X, Y and Z axes.
- abrasive
abrasive
Substance used for grinding, honing, lapping, superfinishing and polishing. Examples include garnet, emery, corundum, silicon carbide, cubic boron nitride and diamond in various grit sizes.
- amorphous
amorphous
Not having a crystal structure; noncrystalline.
- chemical vapor deposition ( CVD)
chemical vapor deposition ( CVD)
High-temperature (1,000° C or higher), atmosphere-controlled process in which a chemical reaction is induced for the purpose of depositing a coating 2µm to 12µm thick on a tool’s surface. See coated tools; PVD, physical vapor deposition.
- composites
composites
Materials composed of different elements, with one element normally embedded in another, held together by a compatible binder.
- flat ( screw flat)
flat ( screw flat)
Flat surface machined into the shank of a cutting tool for enhanced holding of the tool.
- gang cutting ( milling)
gang cutting ( milling)
Machining with several cutters mounted on a single arbor, generally for simultaneous cutting.
- hardness
hardness
Hardness is a measure of the resistance of a material to surface indentation or abrasion. There is no absolute scale for hardness. In order to express hardness quantitatively, each type of test has its own scale, which defines hardness. Indentation hardness obtained through static methods is measured by Brinell, Rockwell, Vickers and Knoop tests. Hardness without indentation is measured by a dynamic method, known as the Scleroscope test.
- high-speed steels ( HSS)
high-speed steels ( HSS)
Available in two major types: tungsten high-speed steels (designated by letter T having tungsten as the principal alloying element) and molybdenum high-speed steels (designated by letter M having molybdenum as the principal alloying element). The type T high-speed steels containing cobalt have higher wear resistance and greater red (hot) hardness, withstanding cutting temperature up to 1,100º F (590º C). The type T steels are used to fabricate metalcutting tools (milling cutters, drills, reamers and taps), woodworking tools, various types of punches and dies, ball and roller bearings. The type M steels are used for cutting tools and various types of dies.
- lapping
lapping
Finishing operation in which a loose, fine-grain abrasive in a liquid medium abrades material. Extremely accurate process that corrects minor shape imperfections, refines surface finishes and produces a close fit between mating surfaces.
- metalcutting ( material cutting)
metalcutting ( material cutting)
Any machining process used to part metal or other material or give a workpiece a new configuration. Conventionally applies to machining operations in which a cutting tool mechanically removes material in the form of chips; applies to any process in which metal or material is removed to create new shapes. See metalforming.
- metalworking
metalworking
Any manufacturing process in which metal is processed or machined such that the workpiece is given a new shape. Broadly defined, the term includes processes such as design and layout, heat-treating, material handling and inspection.
- milling
milling
Machining operation in which metal or other material is removed by applying power to a rotating cutter. In vertical milling, the cutting tool is mounted vertically on the spindle. In horizontal milling, the cutting tool is mounted horizontally, either directly on the spindle or on an arbor. Horizontal milling is further broken down into conventional milling, where the cutter rotates opposite the direction of feed, or “up” into the workpiece; and climb milling, where the cutter rotates in the direction of feed, or “down” into the workpiece. Milling operations include plane or surface milling, endmilling, facemilling, angle milling, form milling and profiling.
- physical vapor deposition ( PVD)
physical vapor deposition ( PVD)
Tool-coating process performed at low temperature (500° C), compared to chemical vapor deposition (1,000° C). Employs electric field to generate necessary heat for depositing coating on a tool’s surface. See CVD, chemical vapor deposition.
- physical vapor deposition ( PVD)2
physical vapor deposition ( PVD)
Tool-coating process performed at low temperature (500° C), compared to chemical vapor deposition (1,000° C). Employs electric field to generate necessary heat for depositing coating on a tool’s surface. See CVD, chemical vapor deposition.
- polishing
polishing
Abrasive process that improves surface finish and blends contours. Abrasive particles attached to a flexible backing abrade the workpiece.
- titanium aluminum nitride ( TiAlN)
titanium aluminum nitride ( TiAlN)
Often used as a tool coating. AlTiN indicates the aluminum content is greater than the titanium. See coated tools.
- titanium carbonitride ( TiCN)
titanium carbonitride ( TiCN)
Often used as a tool coating. See coated tools.
- titanium nitride ( TiN)
titanium nitride ( TiN)
Added to titanium-carbide tooling to permit machining of hard metals at high speeds. Also used as a tool coating. See coated tools.
- titanium nitride ( TiN)2
titanium nitride ( TiN)
Added to titanium-carbide tooling to permit machining of hard metals at high speeds. Also used as a tool coating. See coated tools.
- wear resistance
wear resistance
Ability of the tool to withstand stresses that cause it to wear during cutting; an attribute linked to alloy composition, base material, thermal conditions, type of tooling and operation and other variables.
