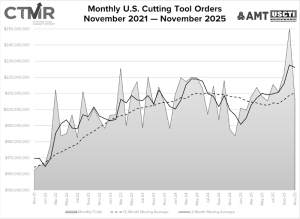
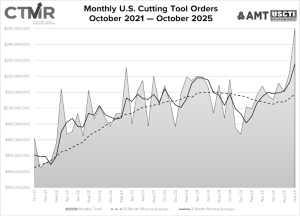
New Mazak Phoenix Technical Center opens for business
Mazak Corporation has opened its new Phoenix Technical Center to provide service and support for manufacturers throughout the Western United States. To showcase the new facility, Mazak will also host a grand opening event later this year. “We continue to establish new facilities to further ensure customers receive the local service and support they need,”...